Technology
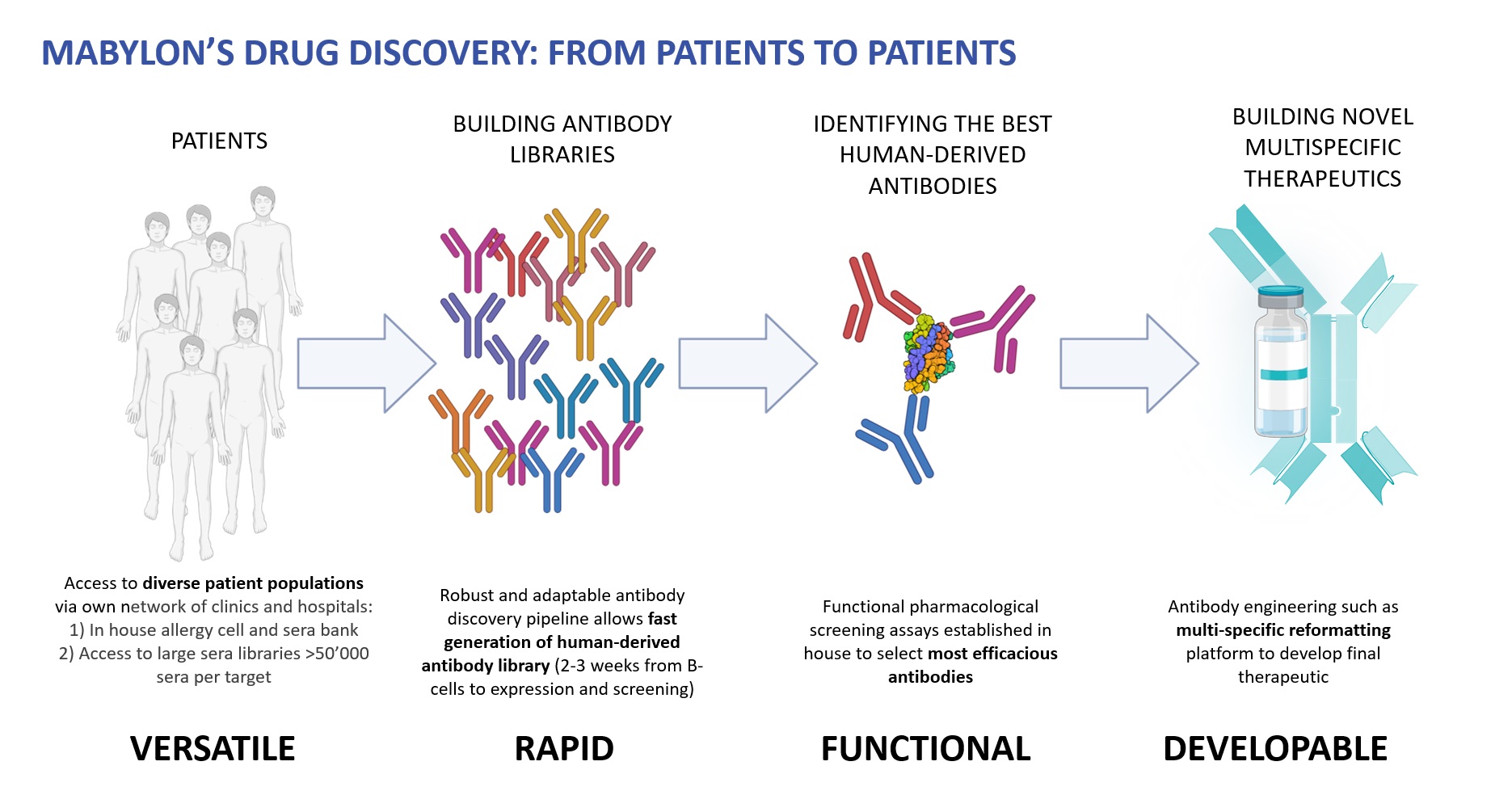
Mabylon’s objective is to discover novel human-derived antibody therapeutics to address several unmet medical needs in the treatment of allergies and neurological disorders.
Our data shows that antibody reactivity against self-proteins is in most cases in the range of 1-10 specific hits every 10,000 patients screened. Mabylon is uniquely positioned to identify even such rare serum reactivities with an unparalleled platform to rapidly screen very large human populations and efficiently clone reactive B-cell clones so to develop novel human-derived antibody therapeutics.
Mabylon is screening large cohorts of individuals giving informed consent to the utilization of surplus blood samples (>100’000 individuals/year) for the presence of autoantibodies against a panel of endogenous antigens. In collaboration with UZH, we avail of a high-throughput pipeline which can screen up to 2’000 patients/day for autoantibodies. Each sample is tested at dilutions ranging across 5 orders of magnitude in a fully automated, robotic workstation. Analyses of binding profiles, affinity estimations and stringent quality controls are performed in real time with customized software developed at the University of Zurich in collaboration with the Swiss Institute of Bioinformatics. Positive hits are being correlated quantitatively to multidimensional matrices of encrypted clinical data including diagnoses, family history, and medication. This unbiased, hypothesis-free approach has the potential to uncover entirely new pathogenetic principles which may not be inferred from prior knowledge. It will also yield novel biomarkers of disease that cannot be discovered by any other methodology.
Mabylon has established the capabilities necessary to execute the work program that we envisage, including IRB submission and approval by the local bioethics authorities (Kantonale Ethikkommission Zurich). Using state of the art B-cell screening technologies and antibody expression platforms, fully human monoclonal antibodies are identified and cloned. Single-cell cloning allows for pairing of the native cognate antibody heavy and light chains. Human monoclonal antibodies are then expressed and purified for testing their therapeutic efficacy in preclinical models of disease. Emerging lead antibodies, with the desired affinity and selectivity, are expected to easily convert into candidate drugs.
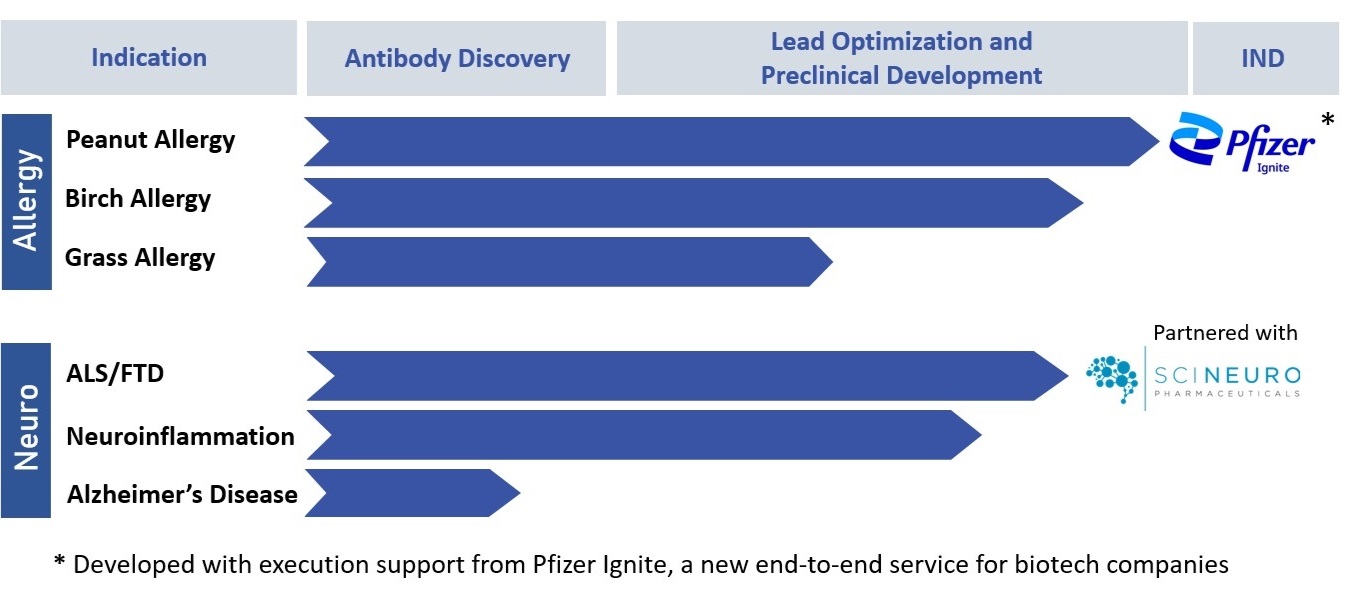
Pipeline
Although our platform can be applied to any therapeutic target of interest, which is accessible to external partners through collaborations, Mabylon’s own R&D pipeline is focused around two therapeutic areas: allergies and neurological diseases, as detailed below.
Publications
1. Review “anti-allergen monoclonal antibodies for the treatment of allergies”. Pengo N, Wuillemin N, Bieli D, Gasser P. Allergo Journal International 2023.
https://doi.org/10.1007/s40629-023-00263-8
3. Multivariate allergen-specific analysis and profiling of serum antibodies from patients with peanut allergy. Paolucci M, Wuillemin N, Köhli A, Ballmer-Weber B, Severin Y, Waeckerle-Men Y, Arena C, Homère V, Bieli D, Kündig TM, Sonati T, Johansen P. Clin Exp Allergy 2023.
https://doi.org/10.1111/cea.14262
4. Targeting Ara h 2 with human-derived monoclonal antibodies prevents peanut-induced anaphylaxis in mice. Paolucci M, Wuillemin N, Homère V, Bieli D, Köhli A, Ballmer-Weber B, Waeckerle-Men Y, Pengo N, Kündig TM, Sonati T, Johansen P. Allergy 2023.
https://doi.org/10.1111/all.15659
5. Strain matters in mouse models of peanut-allergic anaphylaxis: Systemic IgE-dependent and Ara h 2-dominant sensitization in C3H mice. Paolucci M, Homère V, Waeckerle-Men Y, Wuillemin N, Bieli D, Pengo N, Sonati T, Kündig TM, Johansen P. Clin Exp Allergy.

